What Does Glia Do in the Brain? Part 2
Glia – Part of the Brain’s Neuroanatomy: What Is It and What Does It Do?
A Three-Part Series: Part I, Section 2
by Rae Marie Gleason
 Radial Glia is the second big group of astroglial cells, which are bipolar cells each with an ovoid cell body and elongated processes (vine like tendrils that extend from glia and other neuronal cells). They usually have two main processes, one of them forming end feet (small, terminal enlargements of nerve fibers that are in contact with the dendrites or cell bodies of other nerve cells) at the ventricular wall and the other at the pial surface. Radial glia are the first cells to develop from the neural progenitors in the brain; from very early embryonic stages radial glia also form a scaffold which assist in neuronal migration. Radial glia disappear from many brain regions after maturation and transform into stellate astrocytes, although they can be found in the retina (eye).
Radial Glia is the second big group of astroglial cells, which are bipolar cells each with an ovoid cell body and elongated processes (vine like tendrils that extend from glia and other neuronal cells). They usually have two main processes, one of them forming end feet (small, terminal enlargements of nerve fibers that are in contact with the dendrites or cell bodies of other nerve cells) at the ventricular wall and the other at the pial surface. Radial glia are the first cells to develop from the neural progenitors in the brain; from very early embryonic stages radial glia also form a scaffold which assist in neuronal migration. Radial glia disappear from many brain regions after maturation and transform into stellate astrocytes, although they can be found in the retina (eye). The retina contains specialized radial glia called Müeller cells, which make extensive contacts with retinal neurons. The majority of Müeller cells have a characteristic morphology extending longitudinal processes cells along the line of rods and cones in the retina. They occupy up to 20% of the overall volume in the human retina. They also form contacts with a clearly defined group of neurons organized in columnar fashion; a single Müeller cell supports ~16 neurons in the human retina and up to 30 in rodents.
Bergmann glia are specialized semi-radial glia found in the cerebellum. They have small cell bodies and 3-6 processes that extend from the Purkinje cell layer to the pial surface. In early development they have contacts to the ventricular surface and are true radial glial cells, but with the development of the granular layer, they acquire the classical morphology of Bergmann glial cells. It is common for several Bergmann glial cells to surround a single Purkinje neuron (large neuron with many branching extensions found in the cortex of the cerebellum of the brain and plays a fundamental role in controlling motor movement) and their processes form an encasement of the Purkinje cell dendrites. The Bergmann glial cell processes are elaborate and form close contacts with synapses formed by parallel fibers on Purkinje neuron dendrites; each Bergmann glial cell provides coverage for up to 8,000 of such synapses.
Many different populations of astroglial cells inhabit other regions of the CNS.
- Velate astrocytes are found in the cerebellum where they form a sheath around granule neurons; each velate astrocytes enwraps a single granule neuron.
- Interlaminar astrocytes are found in the cerebral cortex of higher primates. Their strange characteristic is a long single process that extends from the soma located within the supragranular layer to cortical layer IV.
- Tanycytes are specialized astrocytes found in the periventricular organs, the hypophysis and the raphe part of the spinal cord.
- Pituicytes are astroglial cells found in the neurohypophysis; their processes surround neuro-secretory axons and axonal endings under resting conditions.
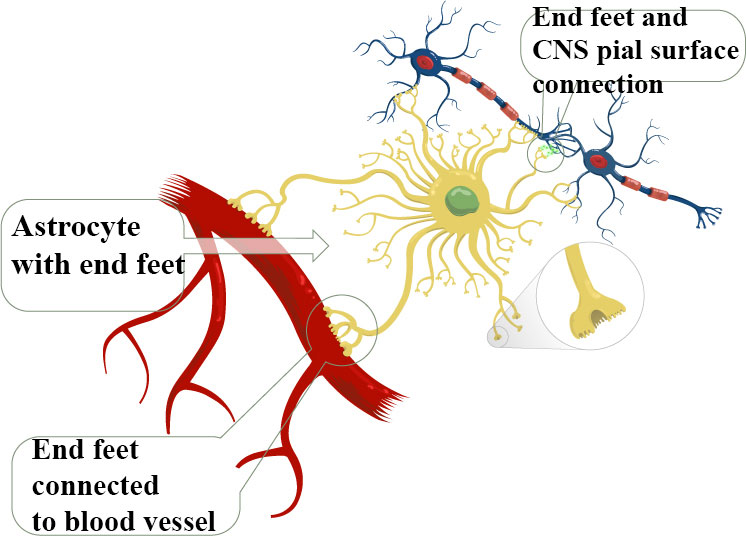
- Perivascular and marginal astrocytes found very close to the pia mater, where they form numerous endfeet with blood vessels and they form the pial and perivascular glia limitans barrier, which assists in isolating the brain parenchyma from the vascular and subarachnoid compartments.
- Ependymocytes, choroid plexus cells and retinal pigment epithelial cells line the ventricles or the subretinal space.
Functions of Astrocytes
Astroglial cell functions are vast. They create the brain environment, build up the micro-architecture of the brain parenchyma, maintain brain homeostasis, store and distribute energy substrates, control the development of neural cells, synaptogenesis and synaptic maintenance and provide for brain defense.Concept of radial glial cells as stem cells
Neurons and macroglia both originate from neuroepithelial cells. In early development neuroepithelial cells transform into radial glia which are now recognized as the neural precursor cell. Asymmetric division of radial glia produce neuronal precursors that migrate to their destinations using the processes of the radial glial as a scaffolding guideline. They also act as progenitors for both astrocytes and oligodendrocytes. Some astrocytes localize in the neurogenic niches of the adult brain, retain the stem cell properties throughout the life span and are the source for the adult neuro-and glio-genesis. Additionally, neuroglial cells are mandatory in promoting neuronal survival at different developmental stages through the release of numerous neurotrophic factors (i.e., epidermal growth factor, EGF, glial cell-derived neurotrophic factor, GDNF, etc.).
Astrocytes define the brain micro-architecture
In the mammalian brain the astroglial cells define the micro-architecture of the parenchyma by dividing the grey matter (through the process known as “tiling”) into mostly independent structural units. The protoplasmic astrocytes occupy their own territory and create the micro-anatomical domains within the limits of their processes. Inside the confines of these anatomical domains the membrane of the astrocytes covers synapses and neuronal membranes, as well as sends processes to plaster the wall of the neighboring blood vessel with their endfeet. The complex astrocytes-neurons-blood vessel is known as a neurovascular unit. Individual astroglial domains are integrated into the superstructure of astroglial syncytia. They also anatomically segregated as they are formed within defined structures; for example in individual barrels of the somatosensory cortex in the brain.
Astrocytes control extracellular K+ homeostasis
Astroglial cells can control extracellular homeostasis which is the tendency of a system, especially the physiological system of higher animals, to maintain internal stability, owing to the coordinated response of its parts to any situation or stimulus that would tend to disturb its normal condition or function, in the brain. By utilizing multiple molecular cascades, astrocytes control concentrations of ions, neurotransmitters and metabolites and regulate water movements. Another function of astrocytes is the control of K+, a potassium ion. It is known that neuronal activity leads to an increase in K+ concentration under physiological conditions and even higher levels under pathological conditions.
Astrocytes remove excess extracellular K+ by at least two different mechanisms. “Spatial buffering” is one mechanisms by which K+ is taken up at the site of higher concentration, redistributed within the astrocytes or the coupled astrocyte network and released at sites where it is lower. In retinal Müller cells, this process is termed K+ siphoning. Secondly, they can remove extracellular K+ by an increase in pump activities, leading to an increase in intracellular K+ and water. The glial syncytia and aquaporine channels expressed in astrocytes also play a role in water homeostasis in the brain.
Astrocytes remove excess glutamate
Glutamate is the major excitatory neurotransmitter in the brain of vertebrates. When released in excess or for long-time, glutamate acts as a powerful neurotoxin that triggers neuronal cell death in many acute and chronic brain lesions. In 1907 Italian psychiatrist Ernesto Lugaro predicted this glia cell function of “chemically splitting or taking up” neurotransmitters. It is now known that astrocytes remove the bulk of glutamate from the extracellular space; they accumulate 80% of the glutamate released, whereas the remaining 20% is taken up by neurons. The glutamate transporters are co-transporters which utilize the energy saved in the form of transmembrane Na+ gradient.
Adapted from Network Glia, www.networklia.eu/en/microglia
Image courtesy of https://braintalks.wordpress.com/
Image courtesy of https://braintalks.wordpress.com/






