What Does Glia Do in the Brain? Part 3
Glia – Part of the Brain’s Neuroanatomy: What Is It and What Does It Do?
A Three-Part Series: Part I, Section 3
by Rae Marie Gleason
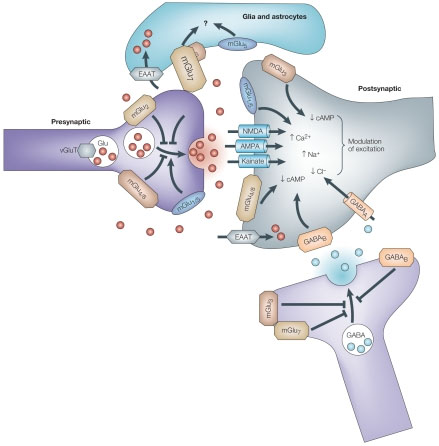 Astrocytes remove excess glutamate. Glutamate is the major excitatory neurotransmitter in the human brain and other vertebrates. When released in excess or for a long-time, glutamate acts as a powerful neurotoxin that triggers neuronal cell death in many acute and chronic brain lesions. In 1907, Italian psychiatrist Ernesto Lugaro predicted this glia cell function of “chemically splitting or taking up” neurotransmitters. It is now known that astrocytes remove the bulk of glutamate from the extracellular space; they accumulate 80% of the glutamate released, whereas the remaining 20% is taken up by neurons. The glutamate transporters are co-transporters which utilize the energy saved in the form of transmembrane Na+ gradient.
Astrocytes remove excess glutamate. Glutamate is the major excitatory neurotransmitter in the human brain and other vertebrates. When released in excess or for a long-time, glutamate acts as a powerful neurotoxin that triggers neuronal cell death in many acute and chronic brain lesions. In 1907, Italian psychiatrist Ernesto Lugaro predicted this glia cell function of “chemically splitting or taking up” neurotransmitters. It is now known that astrocytes remove the bulk of glutamate from the extracellular space; they accumulate 80% of the glutamate released, whereas the remaining 20% is taken up by neurons. The glutamate transporters are co-transporters which utilize the energy saved in the form of transmembrane Na+ gradient.Astroglia control synapotogenisis and synaptic maintenance. Astroglia regulate formation, maturation, maintenance, and stability of synapses, thus controlling the connectivity of neuronal circuits. Astrocytes secrete numerous factors indispensable for synaptogenesis, and without astrocytes, formation of synapses would be greatly depressed. Synaptic formation strictly depends on cholesterol, produced and secreted by astrocytes (that, most likely, provide a building material for new membranes). Additionally, cholesterol may be locally converted into steroid hormones, which in turn can act as synaptogenic signals. Glial cells also affect synaptogenesis through signals influencing the expression of a specific protein, agrin, essential for synapse formation. Another signal by which astrocytes control synaptogenesis is thrombin.
Ultimately, astrocytes control maturation of synapses through several signaling systems, which affect the post-synaptic density, such as controlling the density of post-synaptic receptors. Several distinct soluble factors, released from astroglia affect synapse maturation. These include tumor necrosis factor alpha (TNF-alpha), which regulates the insertion of glutamate receptors into post-synaptic membranes and activity dependent neurotrophic factor (ADNF), which increases the density of NFMDA receptors in the membrane of neighboring postsynaptic neurons.
Because astrocyte membranes can encase the neuronal processes and thus compete with synapses, they may also limit the number of synapses. Additionally, they can be involved in the elimination of synapses in the CNS, the process which underlies the final tuning and plastic of the neuronal inputs. This may be achieved by secretion of certain factors or proteolytic enzymes, which demolish the extracellular matrix and reduce the stability of the synaptic contact. Subsequently, astroglial processes may enter the synaptic cleft and literally close and substitute the synapse.
Neuronal-glial signaling: The concept of the tripartite synapse
In the gray matter, astrocytes are closely associated with neuronal membranes and specifically with synaptic regions, so that astroglial membranes completely or partially enwrap pre-synaptic terminals and post-synaptic structures. Close morphological relations between astrocytes and synapses as well as functional expression of relevant receptors in the astroglial cells prompted the concept of the “tripartite synapse.” According to this concept, synapses are built from three equally important parts, the pre-synaptic terminal, the post-synaptic neuronal membrane and the the surrounding astrocytes. A neurotransmitter released from the pre-synaptic terminal activates receptors in both the post-synaptic neuronal membrane and the perisynaptic astroglial membranes.
This results in the generation of a post-synaptic potential in the neuron and a Ca2+ signal in the astrocytes. This process may also propagate through the astroglial cell body or through the astrocytic syncytium; this Ca2+ signal also triggers release of neurotransmitters from the astrocytes, which in turn will signal onto both pre-and post-synaptic neuronal membranes.
There is still a question regarding whether astrocytes actively participate in the ongoing synaptic transmission. Astroglial signals are on a much slower time scale, rather in the second or even minute range as compared to the rapid signaling of neurons which occurs within milliseconds. Astrocytes therefore can be considered as integrators or modulators. At the same time the intimate coverage of synaptic structures by astroglial membranes can have another important role: the astroglial membranes may effectively isolate synapses and prevent neurotransmitter spillover therefore increasing spatial precision of synaptic transmission.
Synaptic transmission onto astroglia
Receptors located in astroglial perisynaptic processes are activated by neurotransmitters released from presynaptic terminals. In certain regions of the brain, astrocytes receive direct synapse-like (synaptoid) or even classical synaptic connections. For example stimulation or pituitary stalk depolarizes stellate astrocyte-like glia, pituicytes, through direct input from neurons forming synaptoid contacts, where axonal projections end on pituicytes. Likewise, norepinephrine terminals make synaptoid contacts onto septohippocampal astrocytes. In cortex, spontaneous “miniature” excitatory currents were recorded from astrocytes suggesting a very close apposition of glial membranes to the sites of neurotransmitter release.
The concept of “gliotransmission”
Astrocytes and other glial cells can release a variety of transmitters into the extracelluar space. They are currently classified as gliotransmitters although in fact they are the same molecules utilized by neurons such as glutamate, ATP, GABA and D-serine. The first documented description of secretion from astrocytes was made by French neuroanatomist Jean Nageotte, who in 1910 proposed that astroglial cells may release substances into the blood, acting like an endocrine gland. Recently, exocytotic release from astroglial cells was confirmed in numerous experiments which revealed that astrocytes express proteins that are important for exocytosis (the transport of material out of a cell by means of a sac or vesicle that first engulfs the material and then is extruded through an opening in the cell membrane). Additionally glutamate released from astroglia can affect neuronal excitability, possibly modulating synaptic transmission and synchronize synaptic events. However, the role and relevance of gliotransmission for information processing in the brain remains controversial.
Astrocytes in neuropathology
Historically, the pathological potential of neuroglial was recognized at the end of the 19th century and into the beginning of the 20th centuries. Detailed knowledge of the pathological importance of neuroglial in general, and astroglia in particular, has been encumbered evidently by of a long-lasting prevalence of neurocentric views in neurology and neuropathology. That being said, not only are astrocytes fundamental for the control of brain homeostasis, they also represent an important part of the intrinsic brain defense system. Brain insults of multiple etiology (causes) trigger an evolutionary conserved astroglial defense response generally referred to as reactive astrogliosis. This process is essential for both limiting the areas of damage (by scar formation) and for the post –insult remodeling and recovery of neural function.
Astrocytes are common in all types of brain pathologies from acute lesions (trauma or stroke) to chronic neurodegenerative processes (such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and many others) and psychiatric diseases. The pathologically relevant neuroglial processes are many, and they include various programs of activation, which are essential for limiting the areas of damage, producing function. Recent studies also emphasized the role of astroglial degeneration and atrophy in the early stages of various neurodegenerative disorders, which may be important for cognitive impairments. Overall astroglial cells determine to a very large extent the progression and the outcome of neurological disease.
Adapted from: Network Glia, www.networklia.eu/en/microglia
Image courtesy of www.ncbi.nlm.nih.gov
Click here to read Part I, Section 1
Click here to read Part I, Section 2





