Articles on Research
What Does Microglia Do in the Brain? Part II
Microglia: Part of the Brain's Defense and Immune System
A Three-Part Series: Part II, Section 1
by Rae Marie Gleason
This article is a continuation of the NFMCPA Advocate Voice Newsletter Glia Series and an introduction to microglia and its role in the brain.
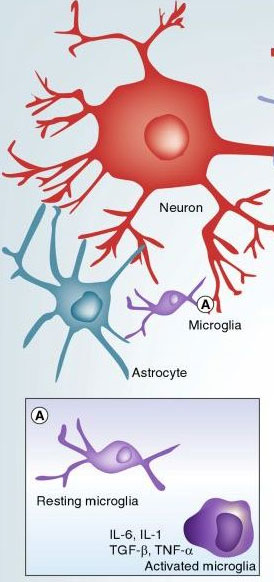 Microglia represents the endogenous brain defense and immune system, which is responsible for the central nervous system (CNS) protection against different types of pathogenic infiltrations. In post natal development in rats the microglia cells immigrate into the brain until pos tnatal day 10. After invading the CNS, the microglia precursors travel throughout the neural tissue acquiring a specific phenotype (biochemical characteristics of an organism), which specifically identifies them as being different from the precursor blood monocytes.
Microglia represents the endogenous brain defense and immune system, which is responsible for the central nervous system (CNS) protection against different types of pathogenic infiltrations. In post natal development in rats the microglia cells immigrate into the brain until pos tnatal day 10. After invading the CNS, the microglia precursors travel throughout the neural tissue acquiring a specific phenotype (biochemical characteristics of an organism), which specifically identifies them as being different from the precursor blood monocytes. "Resting" microglia are the fastest moving cells in the brain
Microglia in the CNS exist in the ramified or ‘resting’ state. They are characterized by a small cell body and elaborate thin processes (extensions), which send out multiple branches and extend in all directions. Every microglia cell has its own territory, and there is very little overlap with neighboring territories. The processes (extensions) of resting microglia cells are constantly moving through their territory; this rapid movement represents the fastest moving structures in the brain. In conjunction with this, microglia processes constantly send out and retract small profusions which are capable of growing and shrinking. They seem to randomly scan their domains. Recent studies have now demonstrated that these processes rest for a period of minutes at sites of synaptic contact. Considering the velocity of this movement, the brain parenchyma (nervous tissue) can be completely scanned by microglial processes every several hours.
The processes motility is not affected by neuronal firing, but it is sensitive to activators such as ATP and inhibitors of purinoceptors (plasma membrane molecules) in the brain. Focal neuronal damage induces a rapid and concerted movement of many microglial processes towards the site of the lesion, and within less than an hour the latter can be completely surrounded by these processes. This injury-induced motility is also affected by activation of purinoceptors and is sensitive to the inhibition of gap junctions which also affects physiological motility of astroglial processes.
It appears that astrocytes signal to the microglia by releasing ATP (and possibly some other molecules) through connexin hemichannels (an assembly of six proteins that can be part of a gap junction between cytoplasm of two adjacent cells). By virtue of receptors residing in the microglial cell plasmalemma, this system can immediately detect injury and initiate the process of active response which triggers the entire microglia activation system.
Microglia activation
When an insult to the brain is detected by microglial cells, a specific program is launched that results in the gradual transformation of resting, ramified microglia into an amoeboid form (changeability of form and means of locomotion); this is generally referred to as ‘microglial activation’ which proceeds through several steps. In the beginning stage, resting microglial retract their processes, which become fewer and thicker, increase the size of their cell bodies, change the expression of various enzymes and receptors, and begin to produce immune response molecules. Some of the microglia cells return into a proliferative ode, and microglial numbers around the lesion site start to multiply. They become mobile and use amoeboid-like movements to gather around sites of insult.
If the damage persists and CNS cells begin to die, microglial cells undergo further transformation and become phagocytes (cells that engulf and absorb waste material, harmful microorganisms, or foreign bodies). This is an abbreviated explanation of microglia activation and the process is simplified for better comprehension by lay people. The process of activation is gradual, and many sub-states exist on the way from resting to phagocytic microglia. Additionally, microglial cells may display various properties in different types of pathologies in different areas of the brain.
Science is still trying to understand the precise nature of the initial signal that triggers the process of microglial activation. It might be associated with either withdrawal of some molecules (‘off-signal’) released during normal CNS activity, or by the emergence of abnormal molecules or irregular concentrations of otherwise physiologically present molecules (‘on-signal’). Either type of signaling can provide microglia with information about the status of the brain with their territorial domain.
The ‘off-signals’ may indicate deterioration in neural networks that are not yet fully characterized. Neurotransmitters are an example for this type of communication. Microglial cells express a variety of classical neurotransmitter receptors such as GABA, glutamate, dopamine, noradrenalin. Most often activation of the receptors counteracts the activation of microglial cells with respect to acquiring a pro-inflammatory phenotype. Possibly, depression of neuronal activity could affect neighboring microglia, turning them into an altered state. The off-signals allow microglia to sense disturbance even if the nature of the damaging factor cannot be identified.
The ‘on-signaling’ is conveyed by an array of molecules, either associated with cell damage or with foreign matter invading the brain. Damaged neurons are capable of releasing high amounts of ATP, cytokines, neuropeptides, and growth factors. Many things can be sensed by microglia and trigger activation. It could be that different molecules can activate various subprograms of this routine, regulating the speed and degree of microglial activation.
Different molecules might be capable of activating various subprograms of this routine, regulating the speed and degree of microglial activation. Some of these molecules are known to carry both ‘off’ and ‘on’ signals. An example is that low concentrations of ATP may be indicative of normal on-going synaptic activity, while high concentrations signal cell damage. Microglia are capable of sensing disturbances in brain metabolism, including accumulation of ammonia, which follows grave metabolic failures that can activate microglial cells either directly or via intermediaries including NO or ATP.
Migration and motility
Microglial migration is essential for many pathophysiological processes, including immune defense. These cells show two types of movement activity.
1. In the ‘resting’ form, they actively move their processes, without translocation of the cell body as already described.
2. In the amoeboid form, microglial cells not only move the processes, but the entire cell can migrate through the brain tissue. The migration occurs in development, when invading monocytes disseminate through the brain.
3. Another type of migration is triggered by a pathologic insult that causes resting microglia to undergo activation. They transform into the amoeboid form and migrate to the site of injury.
Phagocytosis
Microglial cells are the innate phagocytes of CNS tissue. This process is important during brain development, and in pathology and regeneration. In the CNS development microglial phagocytosis is instrumental in removing apoptotic cells and may be involved in synapse removal during development and potentially in pruning synapses in the postnatal brain. This process is intimately involved in many neurological diseases. In response to the lesion, microglial cells accumulate at the damaged site and remove cellular debris or even parts of damaged cells. In Alzheimer’s disease for example, through phagocytosis microglial cells can accumulate different types of pathological factors such as beta-amyloid. In demyelinating neurological conditions such as multiple sclerosis, they can accumulate myelin fragments. Multiple factors, receptors and signaling cascades can regulate microglial phagocytic activity.
Antigen presentation
Microglial cells are the dominant antigen presenting cells in the nervous system. Upon injury the molecules involved in this process are highly up-regulated, and the expression of this complex is essential for interacting with T lymphocytes. This process has been well studied in multiple sclerosis. Microglial cells phagocytose myelin, degrade it and present peptides of the myelin proteins as antigens. By releasing certain cytokines microglial cells are important for recruiting leucocytes in the CNS. They interact with T lymphocytes and, therefore, mediate the immune response in the brain.
Adapted from: Network Glia, www.networklia.eu/en/microglia
Image courtesy of Expert Rev Proteomics, Expert Reviews Ltd. Write comment (0 Comments)





