OHSU/NFMCPA Survey of Symptoms Other than Pain for FDA Meeting Part 2
In preparation for the Food and Drug Administration's Public Fibromyalgia Patient-Focused Meeting December 10, 2013 (rescheduled to March 26, 2014), the National Fibromyalgia & Chronic Pain Association in partnership with Dr. Robert Bennett, Professor of Medicine at Oregon Health & Science University, published a nationwide survey to capture important scientific information to report at the FDA meeting. Robust community participation in the survey during a short time period indicated strong support for more research on fibromyalgia. Dr. Bennett prepared a three-part series of the results for the NFMCPA for education to its constituents and the public. These valuable insights will also be used in further research at OHSU as well as being submitted for publication in a peer-reviewed professional journal. This is Part 2 of that 3-part series.
NFMCPA FDA Survey Shows Successful Alternative Medicine Therapies
Chosen over Unsuccessful Medications by People with Fibromyalgia
Part 2: by Robert Bennett, MD, Professor of Medicine at Oregon Health and Sciences University
During two weeks at the end of November 2013, NFMPCA members looked at a questionnaire designed by Dr. Rob Bennett (Prof. of
 Medicine Oregon Health and Science University). The intent of this questionnaire was to provide information for the Federal Drug Administration (FDA) on common symptoms encountered by fibromyalgia patients, other than pain. This information was to be presented by Jan Chambers, President of the National Fibromyalgia & Chronic Pain Association at an FDA meeting in December 2013, but due to bad weather the meeting was canceled and rescheduled to March 2014. The NFMCPA was able to include data from this survey in the online written comment section of the FDA’s official meeting record. Because it represents information from more than two thousand people regarding specific FDA meeting questions, it holds the potential for a large impact on the total data collected. This survey provided a significant amount of new and important information regarding the suffering endured by people with fibromyalgia.
Medicine Oregon Health and Science University). The intent of this questionnaire was to provide information for the Federal Drug Administration (FDA) on common symptoms encountered by fibromyalgia patients, other than pain. This information was to be presented by Jan Chambers, President of the National Fibromyalgia & Chronic Pain Association at an FDA meeting in December 2013, but due to bad weather the meeting was canceled and rescheduled to March 2014. The NFMCPA was able to include data from this survey in the online written comment section of the FDA’s official meeting record. Because it represents information from more than two thousand people regarding specific FDA meeting questions, it holds the potential for a large impact on the total data collected. This survey provided a significant amount of new and important information regarding the suffering endured by people with fibromyalgia.Just to recap the basic demographics from Part I of the NFMCPA FDA Survey presentation of our data: 3,201 NFMPCA members looked at the questionnaire; it was a rather daunting set of questions which would take 20 to 30 minutes to complete, thus it was not surprising that just over one thousand would-be participants decided to go no further, or started the survey gave up partway through. The good news is that 2187 people completed the survey (see table below).
The majority of those surveyed said they had a diagnosis of fibromyalgia (i.e., only 19 patients reported another chronic pain condition as their primary problem). Recently, criteria have been developed for the use in surveys of this kind that enables an investigator to get a good estimate as to who, in fact, does have fibromyalgia and who does not (using ACR 2010 criteria). Some 1,492 of the original 2,168 patients (69%) had fibromyalgia according to the 2010 ACR criteria. Interestingly, 10 of the 19 patients who did not report a diagnosis of fibromyalgia had a positive diagnosis according to the 2010 ACR criteria.
Age
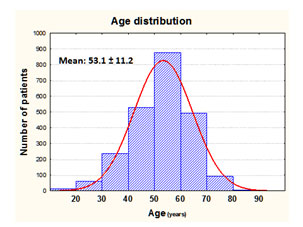
Most of the subjects completing the survey were middle-aged with a mean age of 53. The age distribution can be seen in the accompanying histogram. However, as you can see, there was a normal distribution of ages ranging from the late teens to 80 years old.The major focus of the second part of the survey is an analysis of the various management strategies employed by fibromyalgia patients.
What treatment modalities are used by NFMPCA affiliated patients?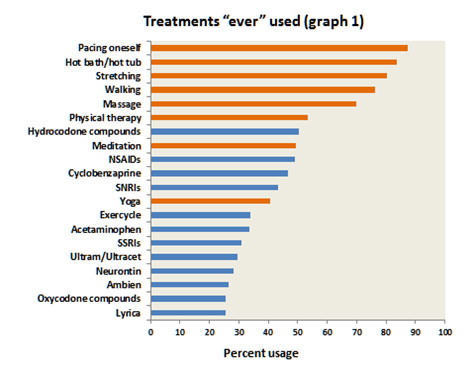
Other common self-help activities (employed by 30 to 50% of patients) were meditation, yoga and use of an exercycle. Common physical modalities rendered by health professionals were massage (70%) and physical therapy (52%).
The most commonly used medications were non-steroidal anti-inflammatory drugs (NSAIDs) and hydrocodone compounds (e.g., Vicodin). The common use of NSAIDs is particularly noteworthy, as clinical studies on ibuprofen and naproxen have failed to show any obvious benefit. However, these studies were done in the late 1980s, and no head-to-head comparisons have been done since then. It is quite likely that NSAIDs are of benefit in those fibromyalgia patients who have concomitant osteoarthritis. This is a very common form of arthritis from middle-age onwards and represents a significant peripheral pain generator that can activate and sustain central sensitization. In these patients use of an NSAID probably helps by indirectly reducing central sensitization.
Cyclobenzaprine (i.e., Flexeril) was used by 47% of patients; this is a muscle relaxant which has been shown to be of benefit in several well conducted studies from 1986 onwards. Its benefit in fibromyalgia patients is mainly due to its positive effect on sleep and pain modulation. In this respect it’s interesting to note that zolpidem (Ambien), a popular short-acting non-benzodiazepine sleeping aid, had been used at one time or another by about 25% of patients. Dr. Robert Bennett’s experience with the use of Ambien in fibromyalgia patients has generally been very positive. There’s one study in the literature showing that Ambien does help sleep, but not pain. The three drugs that have been approved by the FDA for use in fibromyalgia, Savella, Cymbalta (the SNRIs) and Lyrica had been used by less than 48% of patients. Neurontin was used by 28% of patients; this drug has much the same physiological action as Lyrica but is considerably cheaper. The SSRIs (selective serotonin reuptake inhibitors), such as Prozac and Zoloft, were used by just over 30% of patients. This class of drugs does not inhibit the reuptake of norepinephrine; that is, they are not SNRIs (serotonin and norepinephrine reuptake inhibitors).
It appears that the inhibition of norepinephrine reuptake is critical in activating the descending inhibitory pain pathway; hence the SNRIs are more effective in pain reduction. However, the SSRIs are particularly effective in helping patients with mood disorders such as anxiety and depression. Ultram was used by about 30% of patients at some time or another. Ultram is a pain medication which is unique in that it is a dual acting compound; firstly it has opioid-like actions (i.e., acts like morphine) and secondly it has SNRI activity. Several good studies have shown it to be effective for the management of fibromyalgia pain without any overwhelming side effects. The most common problems are mild nausea and/or constipation; these usually resolve over a few weeks. Although it has opioid like actions, it does not have a high addiction potential. In this respect, the FDA has not labeled Ultram as a “controlled substance.” This is relevant to fibromyalgia patients, as physicians more readily prescribed “non-scheduled” medications due to reduced scrutiny by the DEA (Drug Enforcement Administration).
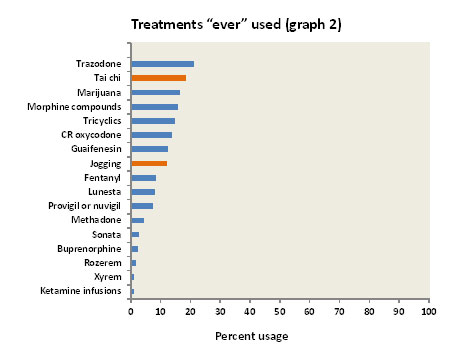
Interestingly, most “fibromyalgia doctors” renounce the use of opioid medications, saying they are generally ineffective. This may be correct, but it is amazing to learn that there has never been a double-blind placebo-controlled study of opioids in fibromyalgia. It seems most unlikely that these opioids would prove to be less effective than a placebo! However, when used over many years the efficacy of opioids tends to wear off, and in some cases they even cause an increase in pain (this is called “opioid hyperalgesia”).
Lunesta, Sonata and Rozerem are all non-benzodiazepine hypnotics. Rozerem is a unique hypnotic in that it is not a sedative; it acts by stimulating melatonin receptors in the hypothalamus and can thus be described as “super melatonin.” Sonata has a very short half-life (one hour) and is useful for patients who wake up in the middle of the night and can’t get back to sleep. On the other hand, Lunesta has a long half-life (six hours) and has the potential to provide a good eight hours of sleep. However, if the patient has to awaken early, Ambien with a half-life of about three hours may be a better choice.
Xyrem (sodium oxybate) is a hypnotic which is FDA approved for the treatment of a rare sleep disorder called narcolepsy. It is been extensively studied in fibromyalgia and found to be effective in helping pain and stiffness as well as sleep. The FDA evaluated Xyrem for use in fibromyalgia in 2010. Although they agreed that it was efficacious in treating fibromyalgia pain, the FDA rejected its approval for use in fibromyalgia on the basis of its potential for abuse (it is virtually identical to the street drug called gamma hydroxybutyrate, with colorful names such as Liquid Ecstasy, Fantasy, Georgia Home Boy, Organic Quaalude, Cherry Menth). Fibromyalgia patients can only obtain Xyrem if it is prescribed “off label.” Many physicians are wary of prescribing “off label” drugs” as there are usually bureaucratic hoops that can prove quite time-consuming. Furthermore, Medicare and insurance companies seldom pay-out on “off label” medications. There is no official price available for non-insurance Xyrem, but it is said to be about $2,000 for a 180 ml (500 mg/ml) bottle.
What medications are used by NFMPCA affiliated patients?
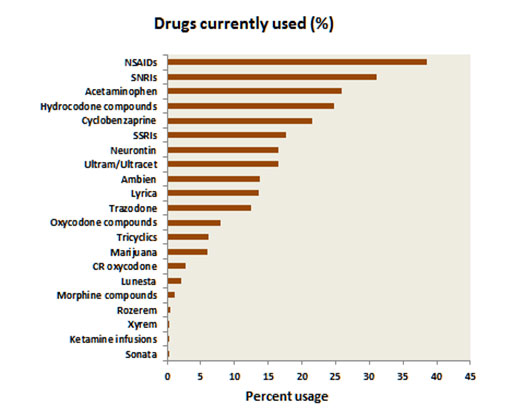 Whereas the last two graphs showed both the pharmacological and non-pharmacological treatments used by fibromyalgia patients, the graph below shows only the drugs used by fibromyalgia patients. The commonest medications used were the NSAIDs. As noted above, these have not been shown to have any effectiveness in treating fibromyalgia pain, but they probably act by ameliorating other sources of pain such as osteoarthritis. The next most common class of drugs was the SNRIs. These include the FDA approved drugs (Savella and Cymbalta), and Effexor, Pristiq and Fetzima. These were followed by acetaminophen (Tylenol) and hydrocodone compounds (e.g., Vicodin). Most of the other drugs in this graph we described in the previous section.
Whereas the last two graphs showed both the pharmacological and non-pharmacological treatments used by fibromyalgia patients, the graph below shows only the drugs used by fibromyalgia patients. The commonest medications used were the NSAIDs. As noted above, these have not been shown to have any effectiveness in treating fibromyalgia pain, but they probably act by ameliorating other sources of pain such as osteoarthritis. The next most common class of drugs was the SNRIs. These include the FDA approved drugs (Savella and Cymbalta), and Effexor, Pristiq and Fetzima. These were followed by acetaminophen (Tylenol) and hydrocodone compounds (e.g., Vicodin). Most of the other drugs in this graph we described in the previous section.
Comparison of “current” drug use and “ever” drug use
The reasons for this discrepancy could be ineffectiveness or side effects, but it may well be the healthcare providers’ reluctance to continue prescriptions of controlled substances. It is interesting to look at the usage of the three FDA approved medications for fibromyalgia. Lyrica had only been used by about 25% of patients with the continued use of just over 10%. On the other hand, the SNRIs (Savella and Cymbalta) had been used by about 40% of patients with continued usage of about 30%.
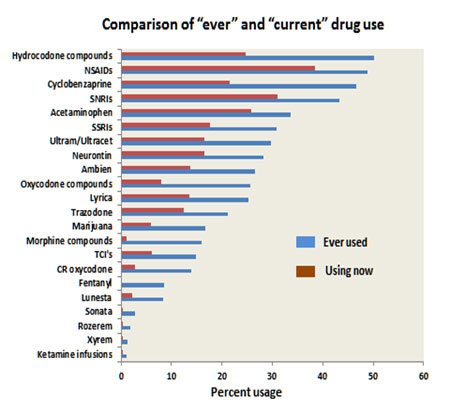 The relatively low use of Lyrica may be explained by the 28% use of Neurontin, which is a similar but much cheaper drug. It is notable that the tricyclic antidepressants (TCI’s) had only been used by just over 10% of patients, with a 5% continuation rate. As noted above, the tricyclic medications were once a mainstay of fibromyalgia drug therapy but have been overtaken by the SNRIs, which are more effective and have fewer side effects.The most efficacious treatment modalities
The relatively low use of Lyrica may be explained by the 28% use of Neurontin, which is a similar but much cheaper drug. It is notable that the tricyclic antidepressants (TCI’s) had only been used by just over 10% of patients, with a 5% continuation rate. As noted above, the tricyclic medications were once a mainstay of fibromyalgia drug therapy but have been overtaken by the SNRIs, which are more effective and have fewer side effects.The most efficacious treatment modalitiesWhy did patients stop taking their medications?
Comparison of 2013 survey with a survey done in 2005.
It is interesting to compare these current results with an Internet study done some nine years ago through the NFA and published in BMC Musculoskeletal Disorders (7th March 2007). The question asked was what treatments have you ever used for management of your fibromyalgia and please rate their effectiveness on a scale of 0 to 100?
As in the current NFMPCA study, self-help strategies (resting, heat modalities, gentle walking and stretching) had been used by over 50% of the patients. The most commonly used medications were over the counter (OTC) pain relievers (61%) such as acetaminophen and ibuprofen, followed by antidepressant medications (56%). Sleep medications (mainly trIcyclics and Ambien) had been used by just over 50%. The most efficacious treatment was prescription sleep medications (rated at 68 out of 100) followed by resting, heat modalities prescription pain medications, antidepressants, massage and pool therapy (all rated at about 60%). The SNRIs, which were the most commonly used medications (after NSAIDs) in the NFMPCA survey, were not rated in this 2005 survey, due to lack of approval by the FDA at that time. The first drug approved by the FDA for use in fibromyalgia was Lyrica (in 2007) followed by Cymbalta (2008) and Savella (2009). It is interesting to observe the rapidly changing drug usage in fibromyalgia, whereas self-help strategies remain common and efficacious.






